Sunday 27 March 2011
Bond Fission
Bond Fission is a fancy term for Breaking Bonds.
Breaking Bonds can either be:
Homolytic
Heterolyric
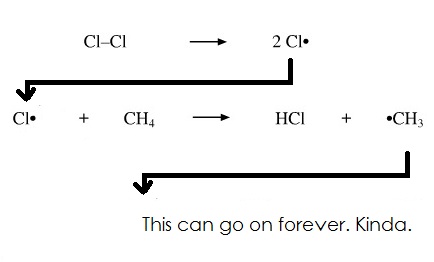
How Radicals form (Kinda, in context of Chlorine):
Breaking Bonds can either be:
Homolytic
The electrons in the bond retract and split in between the molecules to form ions. Wonderful.
Heterolyric
The electrons go to only one molecule, forming a Radical. Dun Dun Duuuuun.Radicals are generally provoked when a bond absorbs an unusual amount of energy, such as UV energy, this is why this happens quite a bit in the upper Stratospheric zone. This relates to Ozone damage et cetera.
(Common in Polar Bonds)
How Radicals form (Kinda, in context of Chlorine):
note: Radicals are bad because they're very reactive, and they can release chain/sequence reactions, deforming naturally occurring bonds, such as O3 (Ozone):
Initiation (When the first radical is made):
Propagation (When a radical reacts to make more radicals):
Termination (When 2 radicals react together to make a stable compound):
Saturday 12 March 2011
Halogenoalkanes
Halogenoalkanes are Hydrocarbon compounds in which one or more hydrogen atoms in an Alkane have been replaced by halogen atoms (Fluorine, Chlorine, Bromine or Iodine).
For example:
Naming Halogenoalkanes:
Name the longest Carbon chain as for branched chain Alkanes.
Carbon atoms bonded to halogen atoms are given the lowest possible numbers.
Halogens are named before alkyl groups :
For more than one of the same Halogen:
di = 2
tri = 3
tetra = 4
For more than one type of halogen, name them alphabetically:
For example:
Naming Halogenoalkanes:
Name the longest Carbon chain as for branched chain Alkanes.
Carbon atoms bonded to halogen atoms are given the lowest possible numbers.
Halogens are named before alkyl groups :
Fluorine atom is named as Fluoro.
Chlorine atom is named as Chloro.
Bromine atom is named as Bromo.
Iodine atom is named as Iodo.
For more than one of the same Halogen:
di = 2
tri = 3
tetra = 4
For more than one type of halogen, name them alphabetically:
Bromo named before Chloro named before Fluoro named before Iodo
Physical Properties of Halogenoalkanes:
The C-Halogen bonds are polar.
The Halogenoalkanes are immiscible in water.
The bigger the chain, the higher the boiling point.
The bigger the Halogen, the higher the boiling point.
The more Halogens, the higher the boiling point.
The more reactive a Halogen, the more stable the compound it makes is.
For example, Fluorine is very reactive. Fluorobutane isn't.
Physical Properties of Halogenoalkanes:
The C-Halogen bonds are polar.
The Halogenoalkanes are immiscible in water.
The bigger the chain, the higher the boiling point.
The bigger the Halogen, the higher the boiling point.
The more Halogens, the higher the boiling point.
The more reactive a Halogen, the more stable the compound it makes is.
For example, Fluorine is very reactive. Fluorobutane isn't.
Chemo-Industrial Calculations
Courtesy of BBC bla bla bla
Chatting about Moles, Atom Economy and Yield.
Friday 4 March 2011
Diploes (Intermolecular Bonding)
Dipolar molecules are any molecules that have a positive end, and a negative end.
When a molecule has a dipole it is polarised.
This is a force that binds molecules together.
This is what guides physical states (Whether something is Solid, Gaseous or Liquid etc.)
There are three types of Dipoles:
1- Permanent Dipoles:
When two atoms sharing a (covalent) bond, and have a really, really, really different electro-negativity (Different charges), they become permanent dipoles, because they're so, so, so attracted to each other. Cute.
HCl has a permanent dipole as Cl has a much higher electronegativity than H.
This is a force that binds molecules together.
This is what guides physical states (Whether something is Solid, Gaseous or Liquid etc.)
There are three types of Dipoles:
1- Permanent Dipoles:
 |
| HCl molecules have a dipole. |
2- Instantaneous Dipoles:
This happens completely randomly.
This is when the electrons in a molecule suddenly move to one side of the "Cloud", instead of being evenly distributed.
When this happens next to other molecules, Induced Dipoles occur!
3- Induced Dipoles:
This happens completely randomly.
This is when the electrons in a molecule suddenly move to one side of the "Cloud", instead of being evenly distributed.
Badass elements: Fluorine and Bromine
 |
| Fluorine is too reactive to be put in glass, this is just an imitation of what Fluorine gas looks like. |
Fluorine is an oxidising agent with an insane charge density.
 |
| Calcium Fluoride physically looks much like Titanium(IV)Oxide. |
Extraction:
CaF + H2SO4 --> CaSO4 + 2HF
Uses include Toothpaste, HCFC making and facial surgery.
Bromine is another halogen, named after "Bromos", greek for "Stench".
Bromine can only be transported in lead tanks, or similar, which are very heavy. Therefore, it's often transported by rail.
 |
| Mmm Gas-y |
Uses include Flame retardants (TBBA), Pharmaceutics, Pesticides, Dyes, Fumigants like Bromoethane.
Industrial terminology
Raw Material: The basic stuff. E.g. Logs, Brine, Rock, Ores...etc
Feedstock: Semi useful things made from the raw materials, such as Chlorine.
BiProduct: Something you accidentally made.
CoProduct: Something you accidentally made, but is useful.
Feedstock: Semi useful things made from the raw materials, such as Chlorine.
BiProduct: Something you accidentally made.
CoProduct: Something you accidentally made, but is useful.
Example:
Making Titanium(IV)Oxide:
Rutile Ore, Rock salt and water ----> Titanium(IV)Oxide, Chlorine and Water ---->Titanium Oxide.
The Rutile Ore, the rock salt and the water are Raw Material/Feedstock. Because it's the input.
Titanium(IV)Oxide is the product. Because it's what you wanted.
Chlorine is the CoProduct. Because it's not what you wanted, but you can still use it for something else.
Extraction of Chlorine
Chlorine lays around in the form of Sodium Chloride.
1- We could use electrolysis to extract it. This is called the "Membrane Cell" method.
Electrolysis is separating 2 substances in an ionic aqueous solution using electric current.
Ions have a charge. When electricity starts running through the aqueous solution, the positive ions get attracted to the cathode (Negative side) and the negative ions get attracted to the Anode (Positive side).
1- We could use electrolysis to extract it. This is called the "Membrane Cell" method.
- Developed in the 1980s.
- Kinda costly because the semi periemable membrane is made of Teflon.
- Low running cost.
- Produces butt loads of Chlorine.
- Less environmental effects than the Mercury Cell.
Electrolysis is separating 2 substances in an ionic aqueous solution using electric current.
Ions have a charge. When electricity starts running through the aqueous solution, the positive ions get attracted to the cathode (Negative side) and the negative ions get attracted to the Anode (Positive side).
Feedstock is Brine
Co products of Chlorine2 are Hydrogen2 and NaOH
Half equations:
2Cl- --> Cl2 + 2e- (Anode)
2H2O + 2e- --> 2OH- + H2 (Cathode)
2- The Mercury Cell is another way of extracting Chlorine.
- Mercury is poisonous.
- The Mercury cell is very effective.
- More costly than the Membrane Cell.
- Uses 1.05g of Mercury per ton of Chlorine.
- Becoming less popular with the Membrane Cell around now.
3- There's another method for extracting Chlorine, the Diaphragm Cell.
Works in the same exact way as a Membrane cell, only that it has a Diaphragm in the middle, as opposed to a Membrane. They're both Semi Permeable textures anyway.
Sunday 27 February 2011
Aqua Regia
One of those things that you stumble upon on the internet, add to your favourites because you think it's interesting and never go back to.
Have a look.
http://en.wikipedia.org/wiki/Aqua_regia
Have a look.
http://en.wikipedia.org/wiki/Aqua_regia
Monday 7 February 2011
Electronic Structure. A bit further detail.
Basically, In GCSE, you were lied to because you were (And probably still are) too dumb to understand the concept that orbitals are fake. Kinda.
Electrons are actually held in Subshells within Shells.
So starting from basic to complex:
Electrons are actually held in Subshells within Shells.
Capacity Hierarchy:
Sub-shells > Shells > Orbitals
So starting from basic to complex:
- Shells are the big "Circle" or "Orbit" around the element.
- Sub-shells are smaller shells that form the big Shells.
- The number of Subshells in a Shell = The number of the shell.
Like this!
- For example, Shell 3 (n=3) has 3 sub orbitals.
- Subshells come in different capacities.
'S' can hold 2 electrons.
'P' can hold 6 electrons.
'D' can hold 10 electrons.
'F' can hold 14 electrons.
- Orbitals are the very small thing. A part of a subshell. There is a maximum of 2 electrons held in each orbital.
In summary, this is the hierarchy of electronic configuration of Sub-shells:
For example (Putting this into use)
The electronic sub-shell configuration of Gold:
1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p6, 4d10, 4f14, 5s2, 5p6, 5d10, 6s1
Where as the first number (Before the letter) is the Energy Level.
Where as the second number (After the letter) is the Number of Electrons held in the subshell.
Putting it into order to make it look tidy:
The things this can tell us:
Friday 28 January 2011
Halogens
The Halogens are 5 non metallic group 7 elements in the periodic table. They have slightly different properties because of their electronic setting (having 7 electrons in their outermost shells).
In terms of patterns and trends, as you move further down the group, electronegativity and reactivity decreases, while boiling points increase. (Exception of Astatine, because that stuff's radioactive).
Halogens have a high electronegativity, and are particularly reactive to form stable ionic crystals (Salts) as the name suggests, halo meaning salt and gen meaning to form.
**Sodium Chloride is the most abundant Halogen-produced Salt**
 |
| Sodium Chloride Crystals |
The charge density of the nucleus of Halogens is important because it shows that they have a high proton charge, which grants them a good electronegativity making them ideal anions, for pairing with the 1st group cations such as Na+.
Halogens, as they have 7 electrons in their outer shells, have an oxidation number of -1, with a few exceptions, for example if it was binded with Oxygen or another Halogen that is more reactive, with Fluorine being the most reactive one.
The difference in reactivity between the group 7 elements is within the fact that they're in consecutive periods, meaning there's a hierarchy relating between the number of outermost shells and the reactivity of the element. This is relating to the idea that more rings around the nucleus make the outer-most shell further away from the centre point of electronegativity, so the pull is spread over a large area decreasing its concentration.
Halogens as oxidation agents follow this general form:
Sunday 23 January 2011
Electrophilic Addition
Electrophilic addition is basically this:
As the chilling alkene gets shanked by the retarded bond, one of its bonds snaps off. The electron's left hanging there.
And then,
So, we end up with this:
Like when you have a retarded bond that decides to jam itself into an unsaturated alkene. A bit like that relative you never liked.
This happens in some stupid stages you're meant to remember. As if it'll mean anything to you in life.
Stage number one. The retarded bond ATTACKS the chilling alkene.
As the bond's snapped off, the retarded bond makes advantage of this via exploiting the open port, as the following diagram illustrates.
And then we get this:
So, we end up with this:
Subscribe to:
Posts (Atom)