Saturday, 9 October 2010
Nuclear Equations in universal context
Notes on nuclear reactions
· Nuclear reactions are reactions that involve the nucleus, while chemical reactions only involve the electrons in an atom.
· Isotope: Atom with an unusual amount of neutrons.
· RadioIsotopes are isotopes that are radioactive. Those are quite useful in medicine and geology.
· Radio Activity is spountanious!
· Radiation types are Alpha, Beta and Gamma.
Fuel Density
Radio Isotopes
· We can use Radio Isotopes to date rocks in Geology.
· We can use Radio Isotopes in tumour treatment in medicine.
· We can use Radio Isotopes in dating any Carbon-based object. (Carbon 14)
Geology
We can date Igneous rocks using Radio Isotopes. Often Potassium 40. It has the half life of 1.3 × 109 years.
Archean age is not the same as the age of the earth! (Not to be confused)
For Radio Isotope Dating to work:
1. Half life must be known accurately.
2. There must be no movement of isotopes in or out of the rocks since formation.
3. The clock cannot be reset by metamorphism.
Medicine
The ratio of Carbon 12 to Carbon 14 is stable in living organisms. This stops when productivity stops, so we can estimate the time of death.
The half life of Carbon 14 is 5730 years.
We use Gamma to destroy cancerous cells.
Iodine is a radioactive isotope that we can use in medicine, it’s a gamma emitter.
We usually use Gamma in medicine because it’s the least Ionising of three types of radiation, which results in the smallest amount of cell deformation possible.
Nuclear Fusion Summary
· ... Requires a large amount of energy.
· ... Is when lighter nuclei join to make a bigger nucleus.
· High temperatures are needed, similar to those in stars; this is because the nuclei initially repel each other due to magnetic charge.
· The energy released is higher than the energy put in the fusion.
Nuclear Fusion might be slightly confusing at first. In GCSE we were taught balancing equations. Whatever's put in, must come out. While in Nuclear Fusion equations, this isn't true.
For example:
2H1 + 3H1 → 4He2 + 1e0
Half-Life - Decay and all that
... Is the time for half the atoms in a radioactive substance to disintegrate.
... Is useful as a measurement in Medicine and Geology.
... Is what’s measured in Carbon 14 dating.
... Varies greatly from an element to another.
...Is different in various isotopes, even if they belong to the same element.
Enthalpy – Continued
Δ H = Mass of Water x Specific heat capacity x θ of water
E.g.
0.21 g of Ethanol was used to heat 100 g of water.
The water’s temperature increased by 10 Celsius.
Δ H = Mass of Water x Specific heat capacity x θ of water
Δ H = 100 x 4.18 x 10
Δ H = 4.18 Kilo Joules
We can use this method to find out the enthalpy in a mole of the substance:
E.g. Knowing that 0.31 g of Ethanol gave 4.18 Kilo Joules of energy:
We divide by the mass and multiply by the molecular mass of the substance.
Δ H = 4.18 Kilo Joules ÷ 0.31 × 46
Δ H = 620.26 Kilo Joules
n.b. Don’t forget to use BASE UNITS before doing any calculation.
A brief lesson in Greek!
· Delta, Δ is the symbol for “Change” in a value.
· Theta, θ is the symbol for “Change” in a heat value.
· H, is the symbol for Enthalpy!
Recap on Atom Structures
J. Dalton defied the atom.
We only have models of the atoms, no one’s “really sure”.
Atoms have equal amounts of electrons and neutrons, otherwise they’re Isotopes.
Necessary knowledge:
· Netrons have a mass of 1.
· Protons have a mass of 1. And are positively charged.
· Electrons have a mass of 0.00055. And are negatively charged.
Despite that electrons are what’s more involved in chemical reactions, chemists are also interested in the nucleus, where protons and neutrons are, because they define and guide what the element of the atom is, or if it’s an isotope.
Isotopes have different amount of neutrons, so they have the same charge as their father element. So, not to be confused with ions.
Some Isotopes are referred to as Radio Isotopes, because we use them in Radiology examinations, as they can be digested and tracked around the body. Examples include 48Calcium & 90Strontium.
- Periodic table guide!
Protons and electrons are the small number.
Neutrons is the big number, take away the small one. If not equal to small number, then it’s an isotope.
Q. What is the relative atomic mass of Chlorine, if it contained 75% Cl35 and 25% Cl37?
((75 x 35) + (25 x 37)) / 100 = 35.5
Activation Enthalpy (Setting stuff on fire)
Cyclo Alkanes
Developing Fuels: Unorthodox Hydrocarbons
Starter Exercise: Calculating the molecular mass
Standard Hydrocarbons
| Molecular Formulae | Hydrocarbon fuel |
| C H4 | Methane |
| C2 H6 | Propane |
| C3 H8 | Ethane |
| C4 H10 | Butane |
| C5 H12 | Pentane |
| C6 H14 | Hexane |
| C7 H16 | Heptane |
| C8 H18 | Octane |
| C9 H20 | Notane |
| C10 H22 | Decane |
N.b. An Isomer is a compound with a standards molecular formulae with an unorthodox structural formulae.
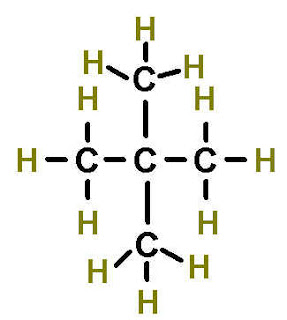
| Pentane | Dimethylpropane (neoPentane) |
History of the periodic table - As much as an A level student needs to know.
• Antoine Lavoisier: Wrote the first extensive list of elements containing 33 elements.
• Distinguished between metals and non-metals. 1770 - 1789
• Jons Berzelius: Developed a table of atomic weights. Introduced letters to symbolize elements. 1828
• Johann Dobereiner: Developed "Triads". Groups of 3 elements with similar properties. 1829
• John Newlands: Arranged the known elements in order of atomic weight, and proposed the Law of Octaves. 1864
• Lothar Meyer: Compiled a periodic table of 56 elements based on the periodictivity of properties such as molar volume, then arranged in order of atomic weight. 1869
• Demitri Mendeleev: Produced a table based on atomic weights, but arranged periodically with elements with similar properties under each other, and left gaps for elements not yet discovered. 1869
• William Ramsay: Discovered the Noble Gases. 1894
Reliability, Precision, Accuracy and Validity
| | What is it? | How can it be applied in an experiment? |
| Reliability | Others can repeat your experiment and get the same reulsts. | This can be attained by repeating the experiment. |
| Precision | How small a measurement is. | This can be attained by using measuring instruments of a smaller scale, e.g. Vernier Calliper vs. Ruler. |
| Accuracy | How close a measurement is to the true value. | This can be attained by reducing all errors. |
| Validity | You can only make a conclusion if your measurement if your measurement have been affected by an independent variable. | This can be attained by carrying out a fair test. |