Breaking Bonds can either be:
Homolytic
The electrons in the bond retract and split in between the molecules to form ions. Wonderful.
Heterolyric
The electrons go to only one molecule, forming a Radical. Dun Dun Duuuuun.Radicals are generally provoked when a bond absorbs an unusual amount of energy, such as UV energy, this is why this happens quite a bit in the upper Stratospheric zone. This relates to Ozone damage et cetera.
(Common in Polar Bonds)
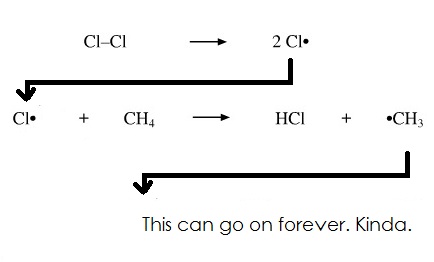
How Radicals form (Kinda, in context of Chlorine):
note: Radicals are bad because they're very reactive, and they can release chain/sequence reactions, deforming naturally occurring bonds, such as O3 (Ozone):
Initiation (When the first radical is made):
Propagation (When a radical reacts to make more radicals):
Termination (When 2 radicals react together to make a stable compound):


No comments:
Post a Comment