MgCO3 (s) + H2SO4 (aq) --> MgSO4 (aq) + CO2 (g) + H2O (l)
Wednesday, 17 November 2010
Epsom Salts
Salts used by Victorians as a medical remedy. They're Magnesium Sulphates, made by bonding Magnesium Carbonate and Dilute Sulphuric Acid.
Alternative Fuels
- Ethanol and Bio Diesel
E.g. From Sugar Canes via fermentation.
Pro - Some CO2 is captured and 'reused'.
Pro - Reduces the demand for fossil fuel.
Con - More expensive than fossil fuels.
Con - Plants are fragile.
Con - Ridiculous land needed to provide the amount of fuel required.
- LPG - Liquefied Petroleum Gas
60% Propane, 40% Butane
Pro - Less CO, NO, and unburnt fuels.
Pro - Cheaper as compared with common fossil fuels.
Con - Difficult to store.
Con - Greater evaporative emissions.
- Hydrogen
Can be consumed in two ways:
1- Via burning: Like common fossil fuels, producing some NOx and water.
2- Via fuel cells: taking place in a cell rather than an engine, producing pure electricity.
Pro - Little or no pollution.
Pro - Semi infinite source of energy.
Con - Difficult to manufacture and store.
*Can be obtained from water via electrolysis.
- Nuclear Energy
Produced via the breaking down of unstable nuclei.
Pro - Long life span.
Con - Expensive and difficult to build and manufacture.
Sunday, 14 November 2010
Mass Spectrometry
Used to measure the atomic or molecular mass of different particles in a sample.
This is like totally awesome because chemists can demonstrate how badass they are by doing CSI stuff with it.
Now there are many different mechanisms in which Mass Spectrometry can be done through, but because you’re just a dim-witted student, you only need to know one. Funnily enough referred to as “Mass Spectrometers”.
Those things sound like they come from Star Wars, but really if you break them down, they’re just as complex as a toaster. Not that you, as a dim-witted student would know how a toaster works. Did you know that the adjusters on toaster are actually timers in minutes, not power levels which is the common misconception.
Breaking a Mass spectrometer down, you’d find three main parts,
An Ioniser, or if you’re feeling Fat and Patriotic, you could call it an Ionizer. All the same: The ioniser is where the sample goes after being injected from a sample inlet for it to get turned into ions, clue is in the name. This is done by bombarding the little stream of sample with electrons. ELECTRONS EVERYWHERE. Btw, if you put the word “Sea of electrons” you deserve to die, because that’s not acceptable A level Chemistry terminology, but since you’re a dim-witted student, the examiner might let you off.
General formulae for what happens in the ioniser zone of a mass spectrometer:
X(g) + e- à X+(g)+ 2e-
The Analyser, or if you’re a fat patriot, you might know it as the analyzer, is the second component in a Mass Spectrometer.
The crap thing about the analyser for a student is that there are so many different types of it. The one you should bear in mine is the one that measures time of flight of the ions. Basically, ions are accelerated using whatever method, usually a magnetic pulse, so that they’re all strolling with the same level of energy, in the same direction, and the fatter ions take longer than the skinny ions to cross a certain distance. This is called measuring the “Time of flight”.
After the ions go through the analyser, and get all analysed and so forth, they go into the Detector chamber, where ions are deteced by an ion detector. The detector produces a varying electric current depending on the ion it got hit with, and the “computer” registers this as a piece of data which eventually gets compiled into an Abundance/Charge graph, aka Mass Spectra!
Monday, 8 November 2010
Molecular shape structures - As far as an A level student needs to know.
Tetrahedral
(E.g. Methane CH4)
109 degrees apart.
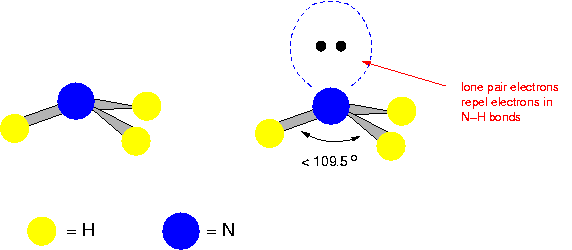
Pyramidal
(E.g. Ammonia)
109 degrees apart.
Bent
(E.g. Water)
104 degrees apart.
Linear
(e.g. BeCl2)
180 degrees apart
Planar Triangular
(E.g. BF3)
120 degrees apart, and two dimensional
Trigonal bipyramidal
(E.g. Phosphorus Pentachloride)
120, or 90 degrees apart
Octahedral
(E.g. Sulfur hexafluoride)
90 degrees apart on all planes.
Quick Guide to Molecule Shapes
A general example in Methane:
Where the firm lines resemble what lies on the 2D plane.
The dotted line resembles what lies backwards.
The Wedge resembles what lies forwards.
Making Methane actually look like this:
We use these denotations because molecules in real life are actually 3 Dimensional.
n.b. Lone pairs are very important in atoms in molecules because they define what else the atom can bond with.
Saturday, 23 October 2010
Blending fuels
This is the process that takes place in an engine (By the piston):
1- Input (Remember as “Suck”)
2- Compression (Remember as “Puff”)
3- Explosion (Remember as “Bang”)
4- Exhaust (Remember as “Blow”)
Knocking in engines occurs when a fuel with a low octane number is used.
Knocking is the pre-ignition of fuel.
Fuel’s ignitability must be in sync with the piston performance, otherwise knocking will occur, damaging the engine.
Octane numbers
Measurements of pre-ignition. (How likely is it for a fuel to pre ignite)
The scale goes from 0 to 100, with some fuels being in the negatives, or even +100.
Example of a 100 is 2,2,4- trimethylpentane.
Example of a 0 is Heptane.
Fraction columns generally produce fuels of 40-60 octane numbers. While modern engines require 95-98. Therefore fuel must be manufactured in order to give it a greater Octane value.
Octane numbers increase as the Hydrocarbon chain becomes shorter, and/or more branched.
We can shorten hydrocarbon chains via Cracking.
E.g. C20 H42 à C8H18 + C12H24
We can increase branching via Isomerism.
We can also use reforming to create cycloalkanes from hydrocarbon chains.
Electronic Structure In Shells
L.O. Define what a shell is.
L.O. Understand how they’re filled.
L.O. Explain and Describe the different types of bonding.
Neil’s idea of Levels became outdated and out-fashioned because it was too simple. This is when the idea of Shells came in.
Shells are groups of orbitals.
This is a more complex concept because the levels idea only includes distance measurements – but no shape or location values …etc
N=1 is the first shell. Contains 2 electrons.
N=2 is the second shell. Contains 8 electrons.
N=3 is the third shell. Contains 18 electrons.
Etc
You can only get S orbitals in n=1.
You can only get S and/or P orbitals in n=2.
You can only get S and/or P and/or D orbitals in n=3.
Etc
| | Atomic Number | N=1 | N=2 | N=3 | N=4 |
| Hydrogen | 1 | 1 | | | |
| Helium | 2 | 2 | | | |
| Lithium | 3 | 2 | 1 | | |
| Beryllium | 4 | 2 | 2 | | |
| Boron | 5 | 2 | 3 | | |
| Carbon | 6 | 2 | 4 | | |
| Nitrogen | 7 | 2 | 5 | | |
| Oxygen | 8 | 2 | 6 | | |
| Fluorine | 9 | 2 | 7 | | |
| Neon | 10 | 2 | 8 | | |
| Sodium | 11 | 2 | 8 | 1 | |
| Magnesium | 12 | 2 | 8 | 2 | |
| Aluminium | 13 | 2 | 8 | 3 | |
| Silicon | 14 | 2 | 8 | 4 | |
| Phosphorus | 15 | 2 | 8 | 5 | |
| Sulfur | 16 | 2 | 8 | 6 | |
| Chlorine | 17 | 2 | 8 | 7 | |
| Argon | 18 | 2 | 8 | 8 | |
| Potassium | 19 | 2 | 8 | 8 | 1 |
| Calcium | 20 | 2 | 8 | 8 | 2 |
| Scandium | 21 | 2 | 8 | 9 | 2 |
| Titanium | 22 | 2 | 8 | 10 | 2 |
| Vanadium | 23 | 2 | 8 | 11 | 2 |
| Chromium | 24 | 2 | 8 | 12 | 1 |
| Manganese | 25 | 2 | 8 | 13 | 2 |
| Iron | 26 | 2 | 8 | 14 | 2 |
| Cobalt | 27 | 2 | 8 | 15 | 2 |
| Nickel | 28 | 2 | 8 | 16 | 2 |
| Copper | 29 | 2 | 8 | 18 | 1 |
| Zinc | 30 | 2 | 8 | 18 | 2 |
| Gallium | 31 | 2 | 8 | 18 | 3 |
| Germanium | 32 | 2 | 8 | 18 | 4 |
| Arsenic | 33 | 2 | 8 | 18 | 5 |
| Selenium | 34 | 2 | 8 | 18 | 6 |
| Bromine | 35 | 2 | 8 | 18 | 7 |
| Krypton | 36 | 2 | 8 | 18 | 8 |
n.b. The Group Number of an element is equal to the amount of electrons in its outermost shell.
| Ionic Bonding | Covalent Bonding | Metallic Bonding |
| A metal, and a nonmetal | 2 non metals | 2 metals |
| Gain or loss of electrons | Sharing electrons | Sharing electrons |
The objective of any bonding process is for atoms to become stable via filling their outermost shells.
Polarity & Electronegativity
The electron pairs shared between two atoms are not necessarily shared equally.
Bond polarity is a useful concept for describing the sharing of electrons between atoms.
A non-polar covalent bond is one in which the electrons are shared equally between two atoms.
A polar covalent bond is one in which one atom has a greater attraction for the electrons than the other atom. If this relative attraction is great enough, then the bond is an ionic bond.
Electronegativity of an atom is its ability to attract electrons to itself.
Dative bonding is when one element in a compound donates both electrons required for the bond to take place.
An example of this is Nitrous Oxide.
Subscribe to:
Posts (Atom)