Tetrahedral
(E.g. Methane CH4)
109 degrees apart.
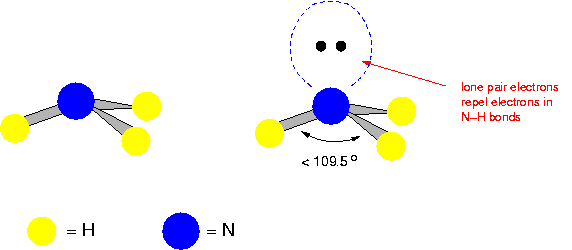
Pyramidal
(E.g. Ammonia)
109 degrees apart.
Bent
(E.g. Water)
104 degrees apart.
Linear
(e.g. BeCl2)
180 degrees apart
Planar Triangular
(E.g. BF3)
120 degrees apart, and two dimensional
Trigonal bipyramidal
(E.g. Phosphorus Pentachloride)
120, or 90 degrees apart
Octahedral
(E.g. Sulfur hexafluoride)
90 degrees apart on all planes.






No comments:
Post a Comment