Chemistry AS portfolio
Sunday, 27 March 2011
Bond Fission
Bond Fission is a fancy term for Breaking Bonds.
Breaking Bonds can either be:
Homolytic
Heterolyric
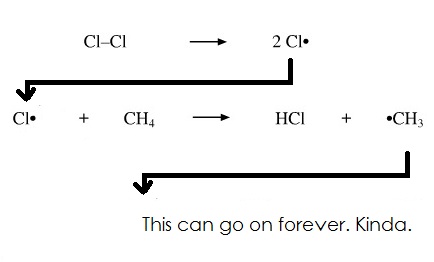
How Radicals form (Kinda, in context of Chlorine):
Breaking Bonds can either be:
Homolytic
The electrons in the bond retract and split in between the molecules to form ions. Wonderful.
Heterolyric
The electrons go to only one molecule, forming a Radical. Dun Dun Duuuuun.Radicals are generally provoked when a bond absorbs an unusual amount of energy, such as UV energy, this is why this happens quite a bit in the upper Stratospheric zone. This relates to Ozone damage et cetera.
(Common in Polar Bonds)
How Radicals form (Kinda, in context of Chlorine):
note: Radicals are bad because they're very reactive, and they can release chain/sequence reactions, deforming naturally occurring bonds, such as O3 (Ozone):
Initiation (When the first radical is made):
Propagation (When a radical reacts to make more radicals):
Termination (When 2 radicals react together to make a stable compound):
Saturday, 12 March 2011
Halogenoalkanes
Halogenoalkanes are Hydrocarbon compounds in which one or more hydrogen atoms in an Alkane have been replaced by halogen atoms (Fluorine, Chlorine, Bromine or Iodine).
For example:
Naming Halogenoalkanes:
Name the longest Carbon chain as for branched chain Alkanes.
Carbon atoms bonded to halogen atoms are given the lowest possible numbers.
Halogens are named before alkyl groups :
For more than one of the same Halogen:
di = 2
tri = 3
tetra = 4
For more than one type of halogen, name them alphabetically:
For example:
Naming Halogenoalkanes:
Name the longest Carbon chain as for branched chain Alkanes.
Carbon atoms bonded to halogen atoms are given the lowest possible numbers.
Halogens are named before alkyl groups :
Fluorine atom is named as Fluoro.
Chlorine atom is named as Chloro.
Bromine atom is named as Bromo.
Iodine atom is named as Iodo.
For more than one of the same Halogen:
di = 2
tri = 3
tetra = 4
For more than one type of halogen, name them alphabetically:
Bromo named before Chloro named before Fluoro named before Iodo
Physical Properties of Halogenoalkanes:
The C-Halogen bonds are polar.
The Halogenoalkanes are immiscible in water.
The bigger the chain, the higher the boiling point.
The bigger the Halogen, the higher the boiling point.
The more Halogens, the higher the boiling point.
The more reactive a Halogen, the more stable the compound it makes is.
For example, Fluorine is very reactive. Fluorobutane isn't.
Physical Properties of Halogenoalkanes:
The C-Halogen bonds are polar.
The Halogenoalkanes are immiscible in water.
The bigger the chain, the higher the boiling point.
The bigger the Halogen, the higher the boiling point.
The more Halogens, the higher the boiling point.
The more reactive a Halogen, the more stable the compound it makes is.
For example, Fluorine is very reactive. Fluorobutane isn't.
Chemo-Industrial Calculations
Courtesy of BBC bla bla bla
Chatting about Moles, Atom Economy and Yield.
Friday, 4 March 2011
Diploes (Intermolecular Bonding)
Dipolar molecules are any molecules that have a positive end, and a negative end.
When a molecule has a dipole it is polarised.
This is a force that binds molecules together.
This is what guides physical states (Whether something is Solid, Gaseous or Liquid etc.)
There are three types of Dipoles:
1- Permanent Dipoles:
When two atoms sharing a (covalent) bond, and have a really, really, really different electro-negativity (Different charges), they become permanent dipoles, because they're so, so, so attracted to each other. Cute.
HCl has a permanent dipole as Cl has a much higher electronegativity than H.
This is a force that binds molecules together.
This is what guides physical states (Whether something is Solid, Gaseous or Liquid etc.)
There are three types of Dipoles:
1- Permanent Dipoles:
 |
| HCl molecules have a dipole. |
2- Instantaneous Dipoles:
This happens completely randomly.
This is when the electrons in a molecule suddenly move to one side of the "Cloud", instead of being evenly distributed.
When this happens next to other molecules, Induced Dipoles occur!
3- Induced Dipoles:
This happens completely randomly.
This is when the electrons in a molecule suddenly move to one side of the "Cloud", instead of being evenly distributed.
Badass elements: Fluorine and Bromine
 |
| Fluorine is too reactive to be put in glass, this is just an imitation of what Fluorine gas looks like. |
Fluorine is an oxidising agent with an insane charge density.
 |
| Calcium Fluoride physically looks much like Titanium(IV)Oxide. |
Extraction:
CaF + H2SO4 --> CaSO4 + 2HF
Uses include Toothpaste, HCFC making and facial surgery.
Bromine is another halogen, named after "Bromos", greek for "Stench".
Bromine can only be transported in lead tanks, or similar, which are very heavy. Therefore, it's often transported by rail.
 |
| Mmm Gas-y |
Uses include Flame retardants (TBBA), Pharmaceutics, Pesticides, Dyes, Fumigants like Bromoethane.
Industrial terminology
Raw Material: The basic stuff. E.g. Logs, Brine, Rock, Ores...etc
Feedstock: Semi useful things made from the raw materials, such as Chlorine.
BiProduct: Something you accidentally made.
CoProduct: Something you accidentally made, but is useful.
Feedstock: Semi useful things made from the raw materials, such as Chlorine.
BiProduct: Something you accidentally made.
CoProduct: Something you accidentally made, but is useful.
Example:
Making Titanium(IV)Oxide:
Rutile Ore, Rock salt and water ----> Titanium(IV)Oxide, Chlorine and Water ---->Titanium Oxide.
The Rutile Ore, the rock salt and the water are Raw Material/Feedstock. Because it's the input.
Titanium(IV)Oxide is the product. Because it's what you wanted.
Chlorine is the CoProduct. Because it's not what you wanted, but you can still use it for something else.
Subscribe to:
Comments (Atom)